What Does Negative Ion Mean In Chemistry
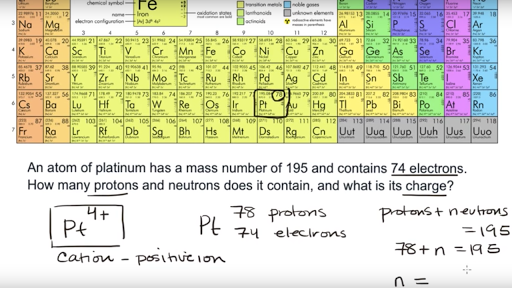
The number of protons plus neutrons in the nucleus 2 electrons 2. This concept is simple enough for small ions.
Introduction To Ions Video Khan Academy
Atom with a net charge caused.
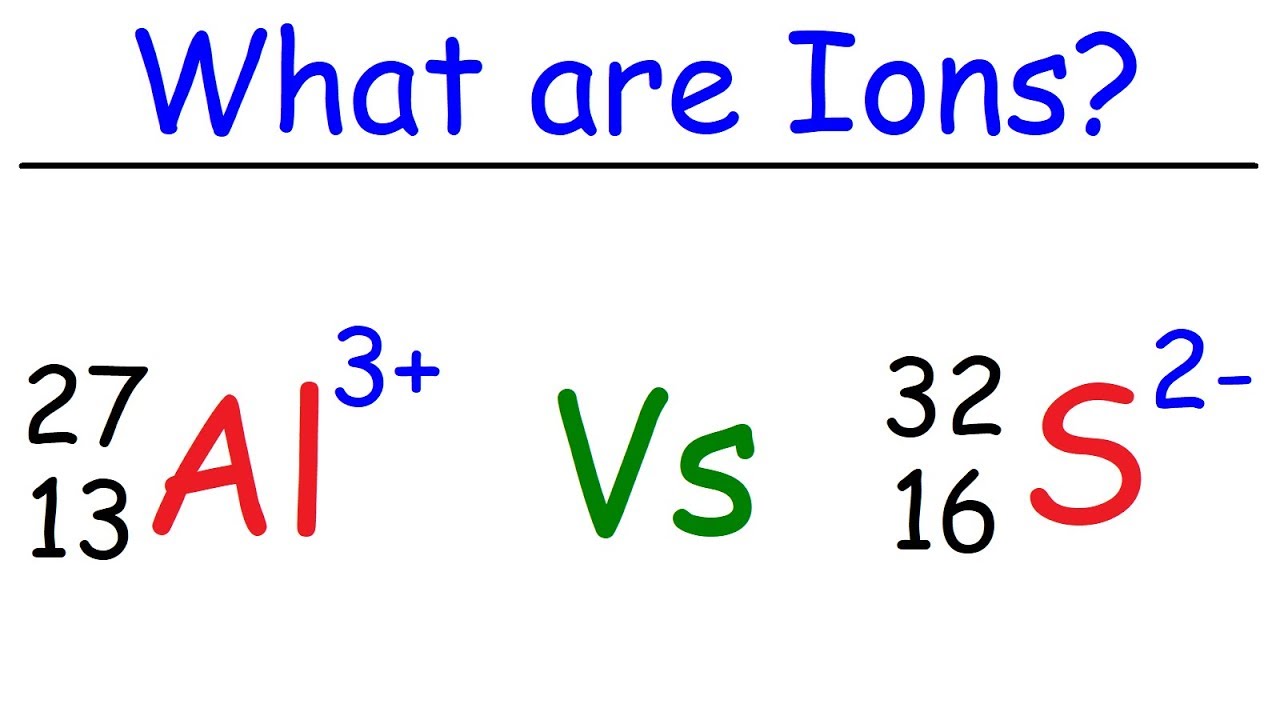
What does negative ion mean in chemistry. Formal charge is the actual charge on an individual atom within a larger molecule or polyatomic ion. It can be monatomic or polyatomic and it can have either a positive or a negative charge. Chloride obviously has a negative charge.
An ion is a charged particle. Atoms and molecules are neutral. It is derived from an atom or a molecule by gain or loss of electrons.
Even the negative charge on the hydroxide oxygen is simple to understand. In ionic substances no molecules exist. Hybrid Resonance The different resonance forms of the molecule help predict the reactivity of the molecule at specific sites.
Symbolized by e- 4 isotope 3. The sum of formal charges on any molecule or ion results in the net overall charge. A carbon with a negative charge is the least favorable conformation for the molecule to exist so the last resonance form contributes very little for the stability of the Ion.

What Are The Two Types Of Ions And How Are They Different A Plus Topper
Difference Between A Positive Ion And A Negative Ion Positive Ion Vs Negative Ion

Ionic Bonding The Science Hive

What Are The Two Types Of Ions And How Are They Different A Plus Topper
Difference Between A Positive Ion And A Negative Ion Positive Ion Vs Negative Ion

Difference Between Positive And Negative Ion Compare The Difference Between Similar Terms

Ion Examples With Positive Negative Charges

What Is An Ion Chemistry Definition
0 Response to "What Does Negative Ion Mean In Chemistry"
Post a Comment